Codonics® becomes exclusive distributor of WasteLog™ in North America
Pharmacolog AB and Codonics partnership agreement creates robust, modern, proven approach to drug diversion
CLEVELAND, OHIO, March 3, 2021. Codonics (www.codonics.com), the global leader of medication management in the perioperative environment, today announced it has signed an agreement with Pharmacolog AB, specialized in developing systems and solutions for more effective and safer use of intravenous drugs, making Codonics the exclusive U.S. distributor of Pharmacolog’s WasteLog™ technology. When integrated with Codonics Safe Label System®, the combined solution provides the most accurate end-to-end diversion control system with a quick and efficient workflow. Together, these technologies will play a critical role in addressing the challenges hospitals face preventing diversion of controlled substances globally.
The stakes for solving, or at minimum mitigating, diversion are high because of risks to those that divert as well as to the entire healthcare system. Complicating an already complex problem are the challenges COVID-19 has introduced. Hospitals are now facing added difficulties caring for COVID-19 patients due to the increased amounts of controlled substances being administered combined with the rapid adjustments to workflow that could contribute to the breakdowns in the normal checks against diversion.
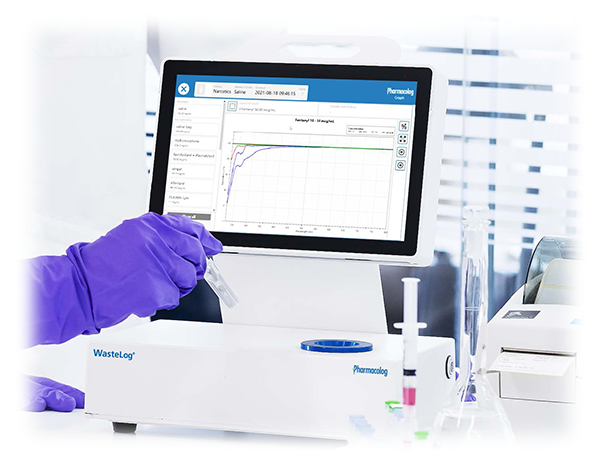 Although Safe Label System and WasteLog™ can be purchased and implemented independently, the integration combines electronic safety checks during medication preparation and administration with better management of the medical waste workflow for a novel approach to drug diversion. In the operating room or wherever medications are prepared, Codonics Safe Label System reads the barcode from parenteral vials and ampoules, provides visual and audible confirmation to ensure drug in hand, then prints a TJC-compliant label that carries the NDC data from that barcode to the prepared syringe. The syringe barcode can be scanned prior to administration for electronic documentation of the medication and concentration including the exact NDC for accurate charge capture and 340B documentation into AIMS/EMR, such as Epic. In Pharmacy or anywhere returns occur, Safe Label System syringes can be scanned enabling WasteLog™ to electronically identify the drug and concentration. Simply injecting a small volume from the syringe (0.3-0.5 mL) into a cuvette enables WasteLog™ to verify the contents. In <3 seconds, WasteLog™ presents a very robust and highly accurate result via the integrated screen. Once the drug is assayed, key data and diversion insight is quickly available for indepth analysis such as trend analysis, report generators and data filters.
Although Safe Label System and WasteLog™ can be purchased and implemented independently, the integration combines electronic safety checks during medication preparation and administration with better management of the medical waste workflow for a novel approach to drug diversion. In the operating room or wherever medications are prepared, Codonics Safe Label System reads the barcode from parenteral vials and ampoules, provides visual and audible confirmation to ensure drug in hand, then prints a TJC-compliant label that carries the NDC data from that barcode to the prepared syringe. The syringe barcode can be scanned prior to administration for electronic documentation of the medication and concentration including the exact NDC for accurate charge capture and 340B documentation into AIMS/EMR, such as Epic. In Pharmacy or anywhere returns occur, Safe Label System syringes can be scanned enabling WasteLog™ to electronically identify the drug and concentration. Simply injecting a small volume from the syringe (0.3-0.5 mL) into a cuvette enables WasteLog™ to verify the contents. In <3 seconds, WasteLog™ presents a very robust and highly accurate result via the integrated screen. Once the drug is assayed, key data and diversion insight is quickly available for indepth analysis such as trend analysis, report generators and data filters.
“We have been focused on establishing strategic partnerships with major international players and are very pleased to have entered into this partnership agreement with Codonics,” said Mats Högberg, CEO, Pharmacolog. “Our partnership provides Pharmacolog with access to Codonics’ existing and growing market and enables us to increase our footprint into this growing healthcare space. The combined solution assures the highest level of safety and compliance will be achieved.”
“We’ve found the ideal partner in Pharmacolog, enabling us to further enhance medication safety and workflow in the pharmacy environment,” said Peter O. Botten, president and CEO of Codonics. “This partnership enables our customers to easily integrate Pharmacolog’s WasteLog™ technology.”



