Mislabeling
Overview
Illegible or Mislabeled Syringes in the Perioperative Environment
Complications from unlabeled or mislabeled syringes has in part prompted the Joint Commission on Accreditation of Healthcare Organizations to create National Patient Safety Goal 03.04.01: “Label all medications, medication containers (for example, syringes, medicine cups, basins), or other solutions on and off the sterile field.”
Unfortunately, illegible or mislabeled syringes are among the operating room’s top three medication errors, accounting for more than 60% of the medication errors made here.
There have been many high-profile cases and national attention given to unlabeled medications and containers by The Joint Commission, the Centers for Medicare & Medicaid Services, the US Food and Drug Administration, the Institute for Safe Medication Practices (ISMP) and others, suggesting that healthcare professionals have basic knowledge of the risks associated with unlabeled containers. Yet, each day, more people die from medical errors than motor vehicle accidents, breast cancer, or HIV1. Put into even more personal terms, every hospital patient may be subjected to as much as one medication error each day.
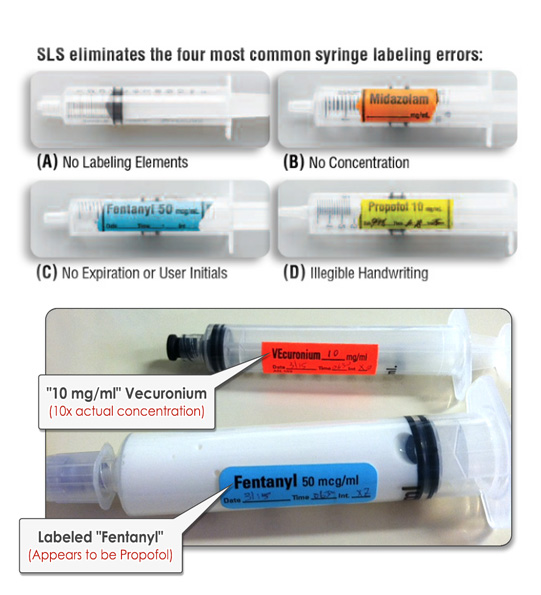
Think about how easy it is to mistake two unlabeled syringes on the OR prep table, both of which contain clear liquid. It’s happened. In fact, it’s happened multiple times and it continues to happen. These errors are human errors — and they are completely preventable.
“Safety is a priority for us. We have to take responsibility for not causing harm to our patients. When there’s not a technology out there, we can think about how nice it would be if it were available, but once you know it’s available, it becomes your responsibility to ensure you’re not causing harm to your patients, knowing there was a technology out there that could have prevented the error but you chose not to implement it.”
–Christina Barnes, PharmD, Director of Pharmacy Services, Avita Health System
Codonics Safe Label System is an award-winning, FDA Class II medication device that improves the safety and accuracy of medication management. A standard of care, SLS is installed in more than 550 leading medical facilities in more than 5000 perioperative locations. SLS is an unprecedented tool in your fight against accidental patient harm, addressing the three most common errors made during the selection, preparation and administration of injectable and intravenous medications, helping to prevent:
- vial and ampoule swaps
- mislabeling and illegible labeling
- syringe swaps
SLS integrates best practices and international standards for accessibility at the point of care, and uses barcode technology to read information from a drug container to electronically verify it against the pharmacy’s hospital-approved drug database. When a drug is scanned on SLS, the clinician is immediately presented with audible and visual confirmation of the drug name and concentration, providing an electronic “double-check” to ensure the medication that was selected is correct. The system then automatically prints a fully compliant, easy-to-read and ready-to-apply label, creating a workflow that prevents illegible and mislabeled syringes, eliminates handwriting and ensures safety and accuracy.
SLS integrates with your existing workflow, adding compliance and pharmacy oversight at every location where on-demand medications are prepared, such as the OR, ICU, PACU, patient floors, and nurse medication prep stations, to deliver safe and predictable results. Safe Label System will help eliminate medication labeling errors and make healthcare safer for everyone.
Join the hundreds of leading hospitals who have prioritized patient safety, committed to bringing preventable medication errors to zero – with Safe Label System.
1 Dhawan Ira, Tewari Anurag, Sehgal Sankalp, Sinha Ashish Chandra. Medication errors in anesthesia: unacceptable or avoidable. Rev. Bras. Anestesiol. Vol.67 no. 2 Mar/Apr 2017
